MyAmici compliance isn’t an afterthought, it’s baked into workflows, records, and approvals in the platform to give confidence in every buying decision, delivery, audit and outcome.

From supplier qualification to material release, MyAmici unifies purchasing, inventory, and approvals in a single validated system. Every transaction is traceable, every record auditable, and every process built to meet the highest regulatory standards, so your lab can move faster, without compromising control.
Validated by design, MyAmici’s GMP Inventory and Purchasing system ensures your business process meets with FDA and MHRA expectations. With embedded documentation, automated audit trails, and controlled approval flows, you’ll always have the evidence you need without the administrative drag.

Gain instant access to Amici’s extensive network of pre-qualified suppliers, each vetted for quality, reliability, and compliance. MyAmici centralizes supplier data, performance records, and ESG credentials, giving you transparent oversight and assurance from day one.

See how our biotech procurement platform simplifies purchasing, saves money, and gives time back to science.
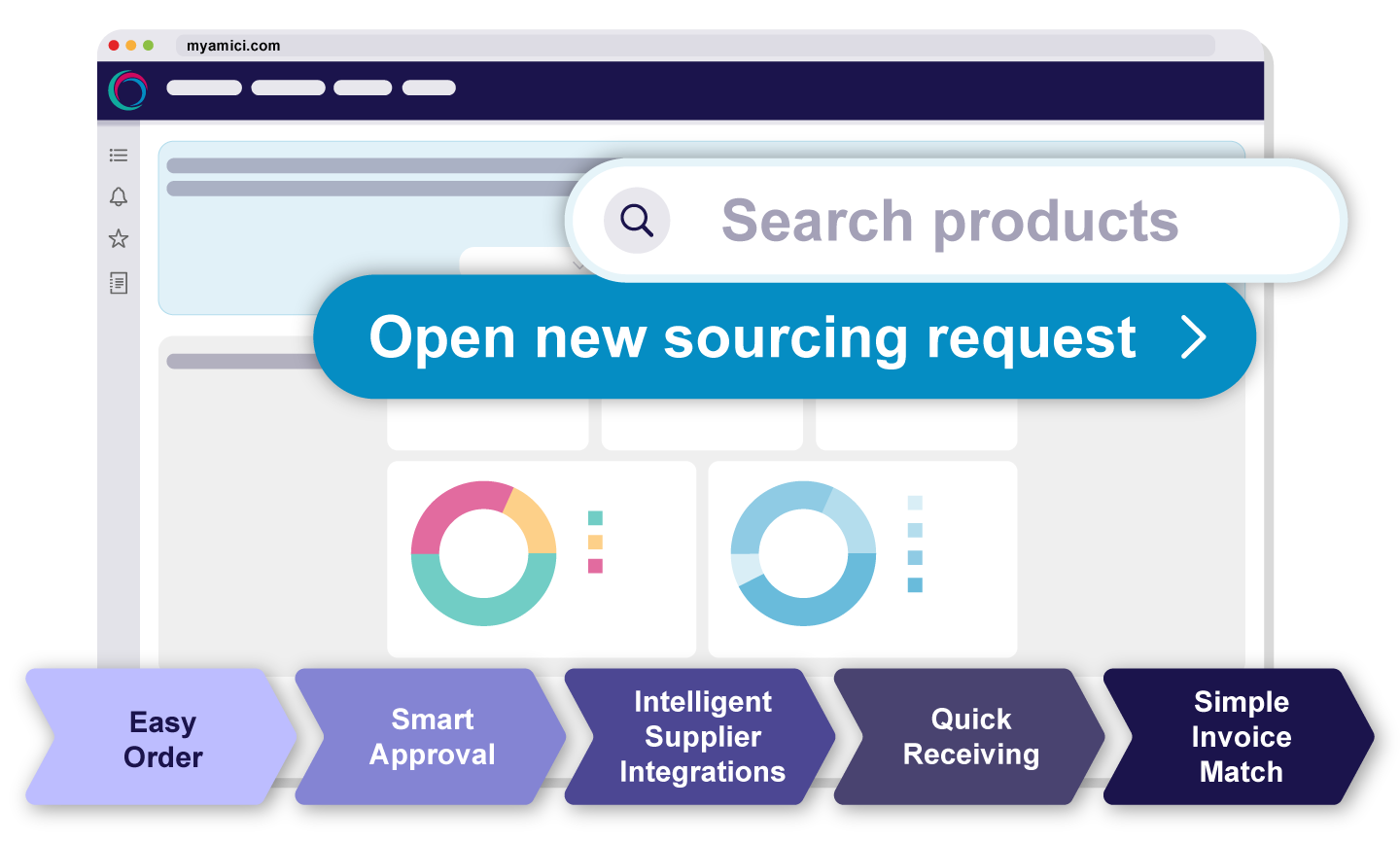
MyAmici enforces compliance across the full procure-to-pay lifecycle. Three-way invoice matching, delegated authority, and exception reporting ensure spend aligns with policy, giving your team the shared visibility it needs to prevent non-compliant purchases before they happen.
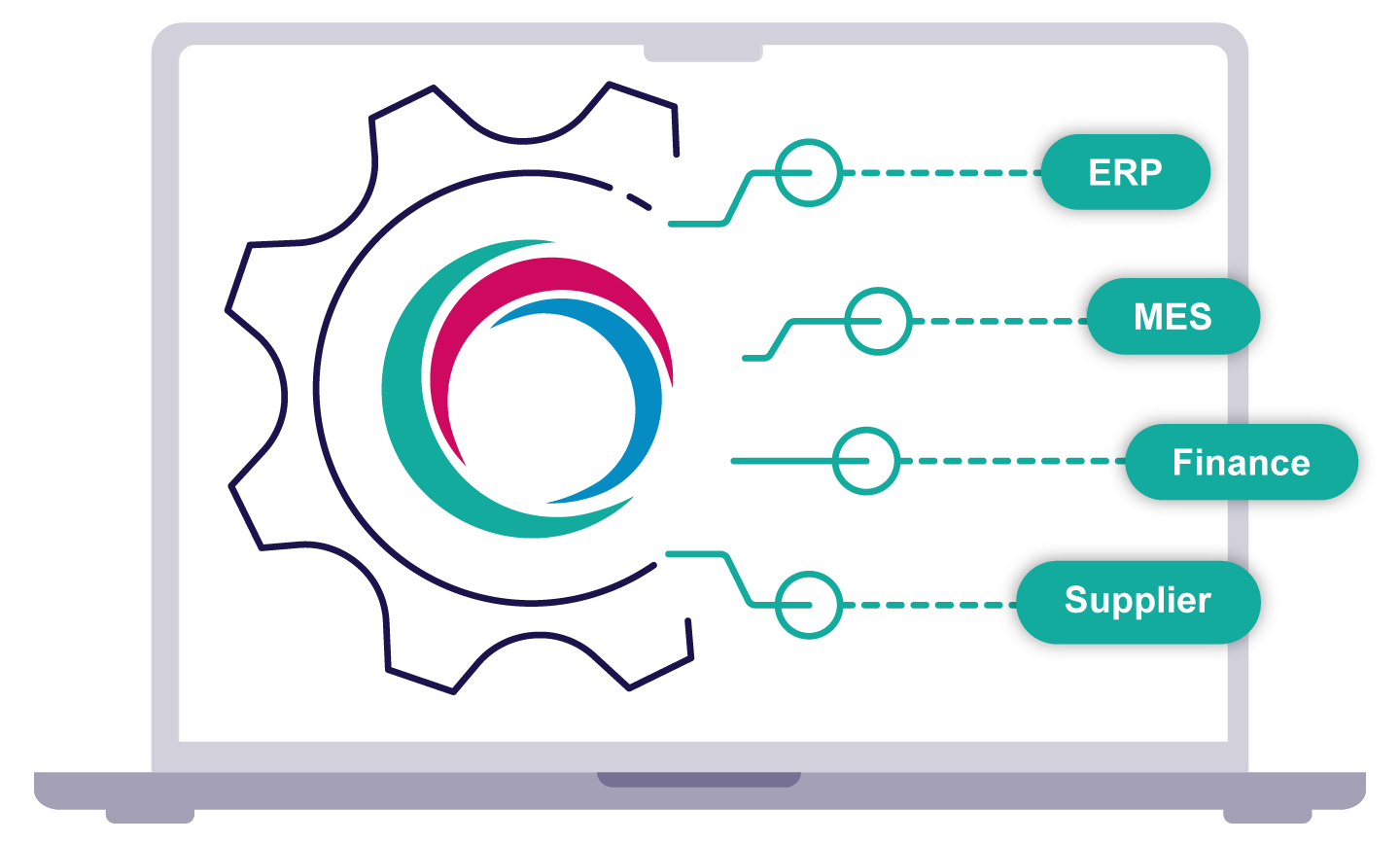
Every MyAmici implementation is fully guided and validated, with ERP integration, test scripts, and IQ and OQ validation packs delivered as standard. Go live in a matter of weeks with documented processes that keep Quality and Compliance aligned from day one.
From sourcing to delivery MyAmici streamlines every step of biotech purchasing.
From lab benches to boardrooms, MyAmici is delivering value where it counts. Dive into our case studies to see how we make it happen.

Dive into a curated mix of thought leadership, industry insight, and behind-the-scenes biotech lab wins.
Yes. MyAmici offers a fully validated GMP Inventory and Purchasing system – audited by the FDA and MHRA. Validation documentation, test scripts, and configuration records are delivered as part of implementation to support compliance with GMP and GxP requirements.
MyAmici captures every transaction, approval, and change with time-stamped audit trails. Built-in approval workflows and version-controlled documentation ensure that data integrity, traceability, and defensibility are maintained at all times – making audits faster and less stressful.
Yes. MyAmici integrates seamlessly with leading ERP and finance systems to keep purchasing, inventory, and quality records aligned. This ensures consistency between operational and financial data – reducing manual reconciliation and the risk of compliance gaps.
MyAmici provides access to a large network of pre-qualified suppliers vetted for quality, reliability, and compliance. Within MyAmici, supplier performance data, certifications, and ESG metrics are tracked and visible, helping QA teams maintain approved supplier lists with confidence.
Implementation timelines vary by module. Purchasing typically goes live within 2–8 weeks, while GMP Inventory implementations take around 2–4 months. Each rollout includes full validation – documented evidence that the system works as intended and meets regulatory requirements for GMP compliance.
See how Amici simplifies procurement, streamlines ops, and saves you money, all in one platform built for biotech.