Ensure patient safety, traceability, and compliance confidence with a fully validated inventory platform built for regulated environments.
Our GMP Inventory solution gives you configurable control across every step of material movement — from inbound inspection to issue, storage, and disposal. Seamlessly connected with purchasing, it’s the fastest, most reliable route to GMP compliance and audit readiness.
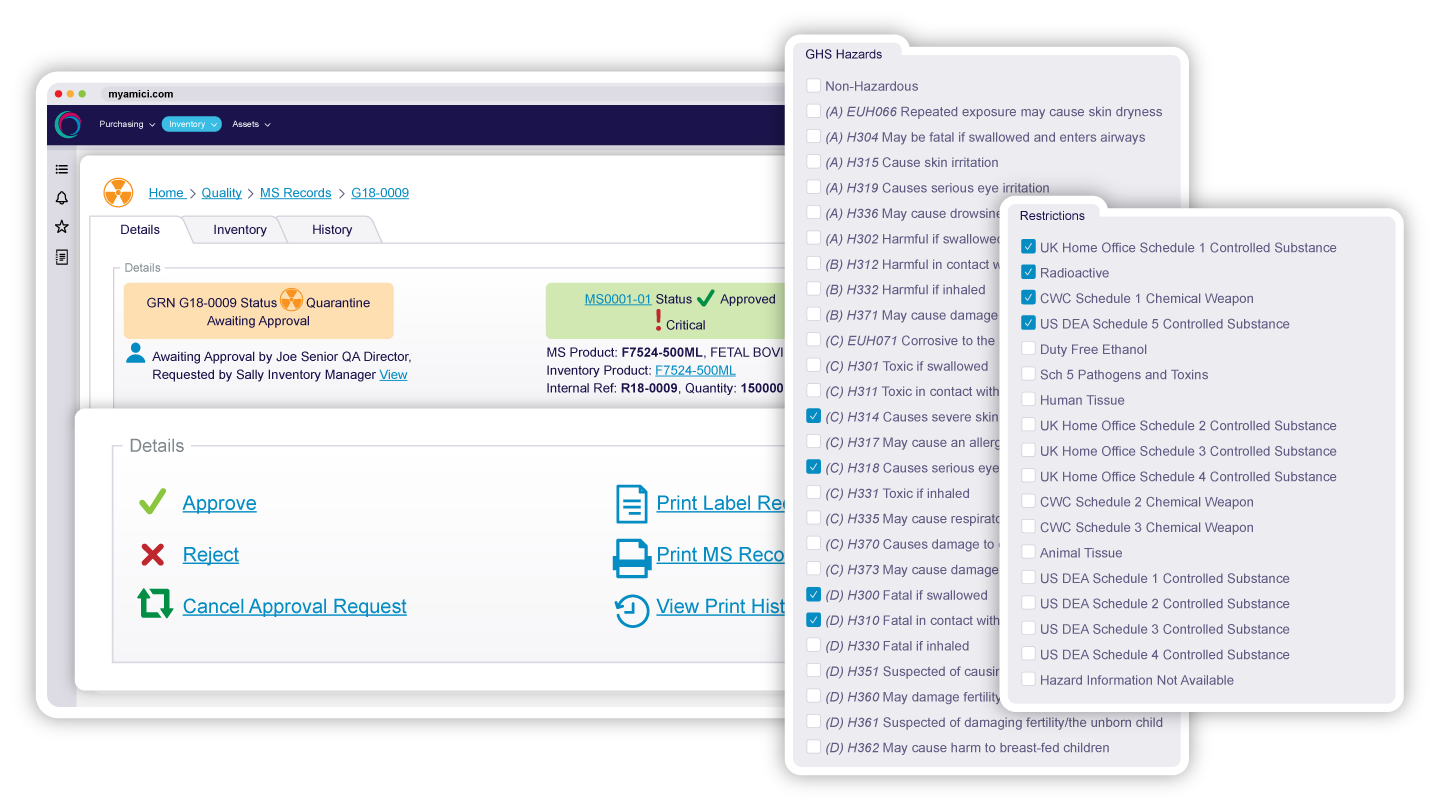
Set material specifications at the master data level to ensure only approved items enter your GMP inventory. Automatic quarantine on arrival prevents unverified materials from reaching controlled areas until inspection and testing are complete, ensuring every item meets defined quality and compliance standards while electronic processes save time and improve the accuracy of record keeping.
Build workflows which strengthen quality whilst optimising process. From low-risk consumables to critical reagents, every inbound item follows a validated, repeatable testing process with the appropriate checks, defined by your own methodology.
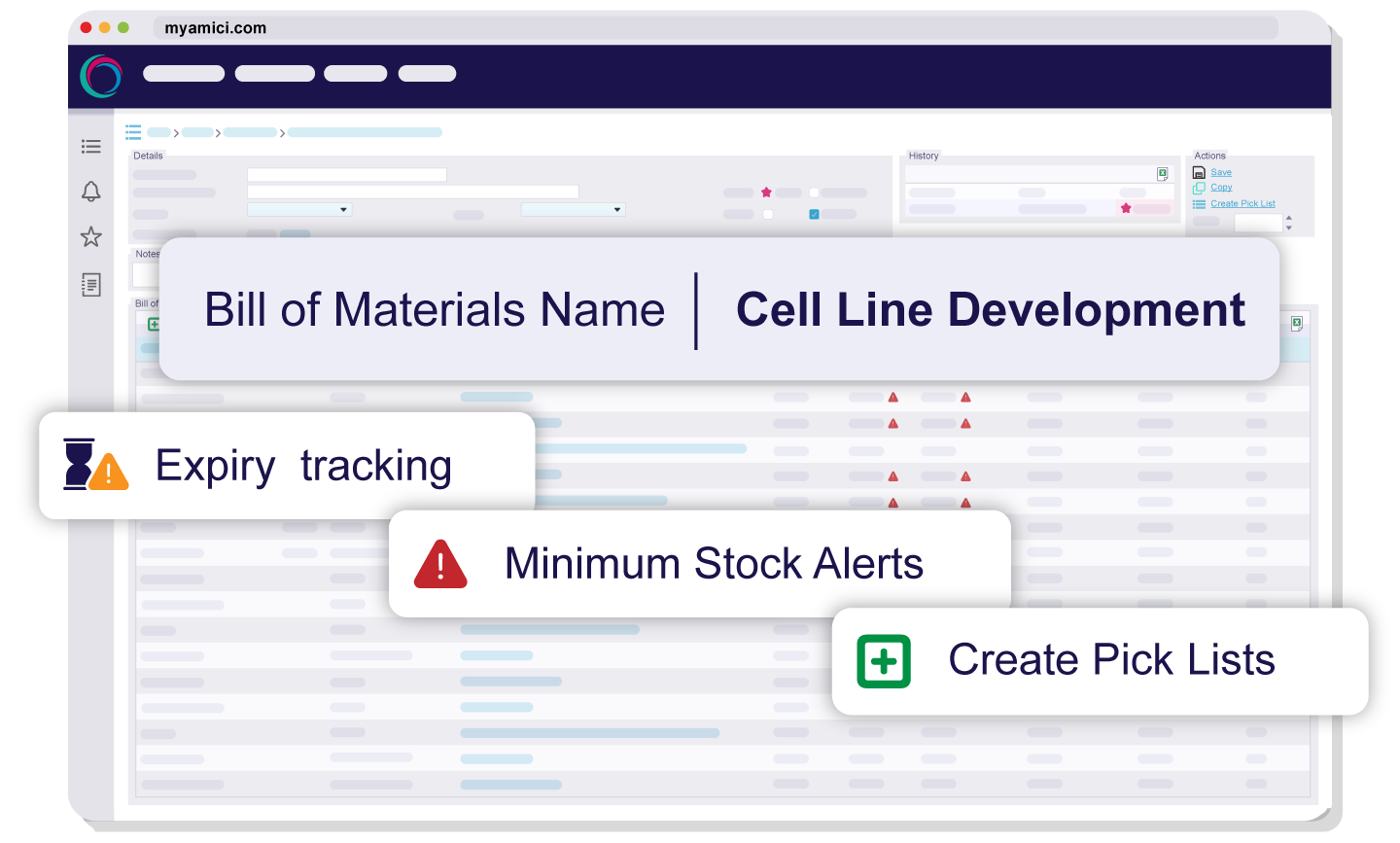
Create Bills of Materials to detail each stage of the process and define raw material requirements, including the ability to yield intermediate products from the BOMs. Plan in advance by generating first expiry, first out (FEFO) picklists in seconds and reserving raw materials for future picking – helping production and stores stay perfectly aligned.
See how our biotech procurement platform simplifies purchasing, saves money, and gives time back to science.

Configure the templates for each label once and effortlessly print your GMP labels. With lot, batch, and expiry control, plus FEFO enforcement, you can instantly locate any material, trace its movement history, and produce audit-ready records in seconds.
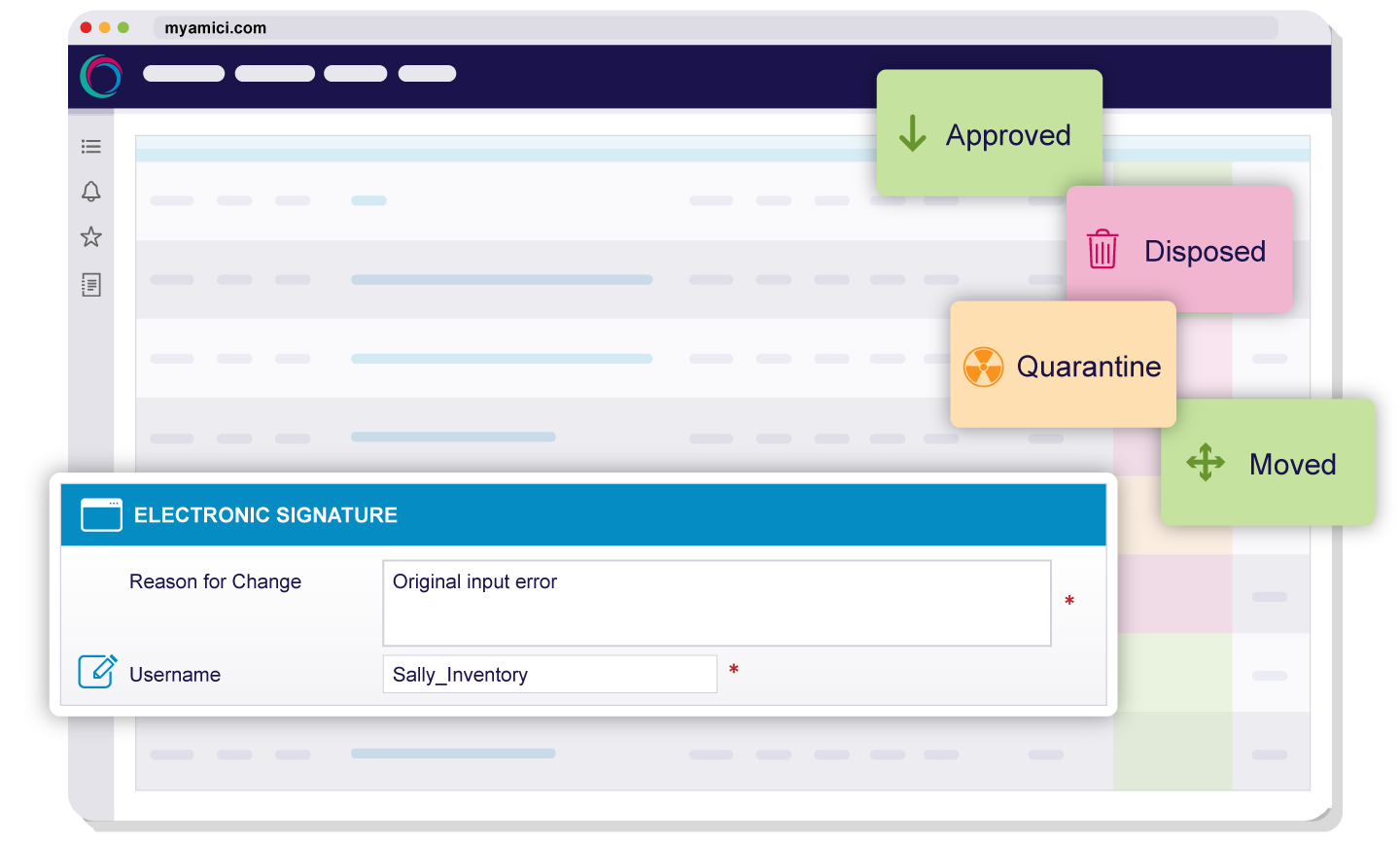
Every user action, transaction, and approval is captured in a secure, time-stamped audit trail. Electronic signatures and role-based permissions enforce accountability while maintaining data integrity across your entire operation.
From lab benches to boardrooms, MyAmici is delivering value where it counts. Dive into our case studies to see how we make it happen.

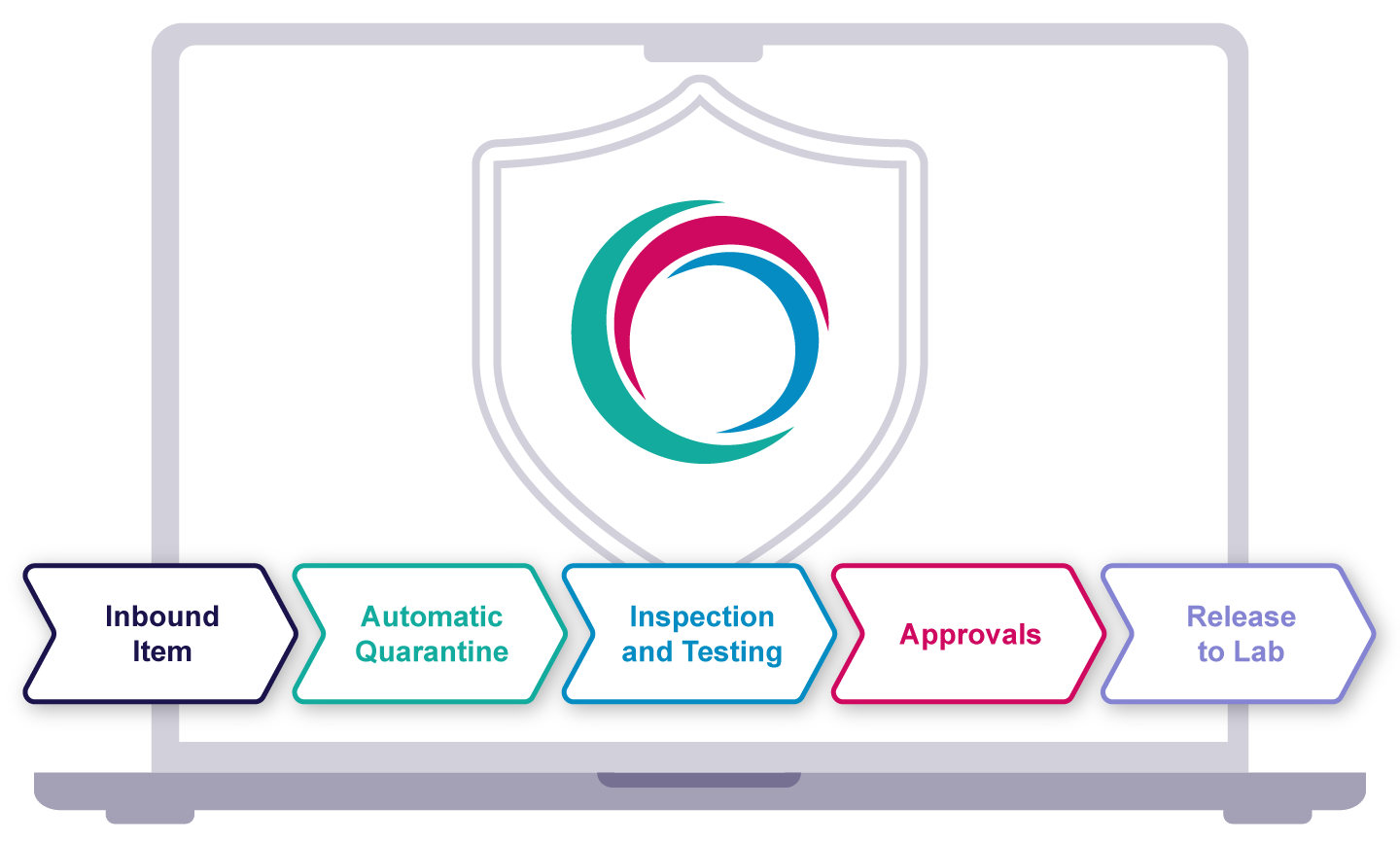
Amici is the only company in the world to offer a configurable SaaS GMP Inventory and Purchasing system. It’s fully validated, regularly audited by the FDA and MHRA, and designed specifically for regulated environments. Unlike generic LIMS or ERP modules, it combines purchasing and inventory in one validated workflow — delivering end-to-end traceability, defensibility, and faster implementation.
Every GMP system deployment includes a comprehensive validation pack that supports IQ, OQ, and PQ. You’ll receive a full dossier — over 3,000 pages of documented test evidence — confirming the software performs exactly as intended. Our team guides you through every stage, so you meet regulatory expectations without slowing down your implementation.
Yes. MyAmici’s architecture is designed to integrate seamlessly with existing ERP, finance, and quality systems through secure APIs. That means you can maintain master data control and financial visibility while gaining GMP-compliant inventory management — without duplicating effort or losing data integrity.
Because MyAmici is a configurable SaaS platform (not customized build), implementation is significantly faster and more cost effective than traditional validated systems. Most GMP deployments go-live in a matter of weeks — not years — supported by dedicated Amici specialists who’ve completed this process many times before.
Our software and validation documentation are kept continuously audit-ready. Updates follow a controlled release cycle, each change fully impact-assessed and tested before deployment. You’ll receive updated validation documentation and support through requalification if needed, ensuring your environment stays compliant long after go-live.
See how Amici simplifies procurement, streamlines ops, and saves you money, all in one platform built for biotech.